Chemists from Rice University and the University of Texas at Austin learned more isn’t usually superior when it arrives to packing charge-acceptor molecules on the surface area of semiconducting nanocrystals.
The mixture of organic and natural and inorganic elements in hybrid nanomaterials can be tailor-made to capture, detect, transform or command light in exceptional means. Desire in these products is higher, and the rate of scientific publication about them has grown a lot more than tenfold above the past 20 many years. For illustration, they could most likely boost the efficiency of photo voltaic electrical power systems by harvesting electrical power from wavelengths of sunlight—like infrared—that are skipped by conventional photovoltaic photo voltaic panels.
To generate the products, chemists marry nanocrystals of mild-capturing semiconductors with “demand acceptor” molecules that act as ligands, attaching to the semiconductor’s floor and transporting electrons absent from the nanocrystals.
“The most-researched nanocrystal units aspect higher concentrations of demand acceptors that are certain immediately to the semiconducting crystals,” explained Rice chemist Peter Rossky, co-corresponding creator of a current study in the Journal of the American Chemical Society. “Commonly, individuals test to improve the area concentration of charge acceptors simply because they assume the charge of electron transfer to continuously increase with area-acceptor concentration.”
A handful of printed experiments had proven electron transfer charges in the beginning boost with floor-acceptor concentration and then drop if floor concentrations proceed to increase. Rossky and co-corresponding creator Sean Roberts , an associate professor of chemistry at UT Austin, understood molecular orbitals of ligands could interact in approaches that could affect charge transfer, and they envisioned there was a position at which packing extra ligands onto a crystal’s floor would give rise to these kinds of interactions.

Rossky and Roberts are co-principal investigators with the Rice-dependent Heart for Adapting Flaws into Features (CAFF), a multiuniversity application backed by the National Science Foundation (NSF) that seeks to exploit microscopic chemical problems in resources to make innovative catalysts, coatings and electronics.
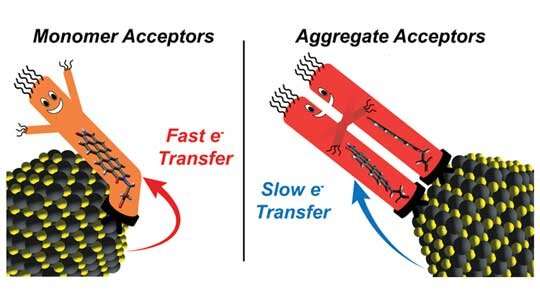
To take a look at their notion, Rossky, Roberts and colleagues at CAFF systematically examined hybrid resources containing lead sulfide nanocrystals and different concentrations of an oft-studied natural and organic dye identified as perylene diimide (PDI). The experiments confirmed that constantly escalating the concentration of PDI on the area of nanocrystals inevitably created a precipitous drop in electron transfer prices.
Rossky claimed the critical to the behavior was the impact that ligand-ligand interactions involving PDI molecules have on the geometries of PDI aggregates on crystal surfaces. Compiling proof to present the affect of these aggregation outcomes needed knowledge from every study team and a watchful mixture of spectroscopic experiments, electronic framework calculations and molecular dynamics simulations.
Roberts claimed, “Our effects display the value of contemplating ligand-ligand interactions when building light-weight-activated hybrid nanocrystal materials for charge separation. We showed ligand aggregation can undoubtedly gradual electron transfer in some conditions. But intriguingly, our computational styles forecast ligand aggregation can also speed electron transfer in other conditions.”
Rossky is Rice’s Harry C. and Olga K. Wiess Chair in Organic Sciences and a professor each of chemistry and of chemical and biomolecular engineering.
Far more details:
Matthew W. Brett et al, The Increase and Future of Discrete Organic–Inorganic Hybrid Nanomaterials, ACS Physical Chemistry Au (2022). DOI: 10.1021/acsphyschemau.2c00018
Danielle M. Cadena et al, Aggregation of Cost Acceptors on Nanocrystal Surfaces Alters Costs of Photoinduced Electron Transfer, Journal of the American Chemical Modern society (2022). DOI: 10.1021/jacs.2c09758
Presented by
Rice University
Citation:
More inbound links aren’t essentially greater for hybrid nanomaterials (2023, January 4)
retrieved 4 January 2023
from https://phys.org/information/2023-01-one-way links-automatically-hybrid-nanomaterials.html
This document is matter to copyright. Aside from any reasonable dealing for the function of personal analyze or study, no
element might be reproduced with out the penned authorization. The information is delivered for facts purposes only.




