Contents
Off-target base editing with a TALE-free DddA split half
We investigated whether one DdCBE subunit alone bound to a target site in the human mitochondrial gene could catalyze C-to-T conversions when paired with a TALE-free DddAtox half with no apparent target site in the mtDNA. To test this hypothesis, we chose a DdCBE pair (L-1397N (the amino-terminal DddAtox half split at G1397 fused to the carboxyl terminus of the TALE array designed to bind to the left half-site) plus R-1397C (the C-terminal DddAtox half split at G1397 fused to the C terminus of the TALE array designed to bind to the right half-site)) specific to the MT-ND1 gene, which catalysed C-to-T conversions efficiently with a frequency of 60.7% ± 3.2% at position 11 (C11) at the target site in HEK293T cells (Fig. 2a). Surprisingly, each subunit alone (L-1397N or R-1397C), paired with the other TALE-free DddAtox half, also induced base editing, albeit less efficiently than did the original pair. Thus, L-1397N alone and R-1397C alone bound to the ND1 target site showed base editing with a frequency of 31% ± 1.6% and 8.1% ± 0.5%, respectively (Fig. 2). In other words, the DdCBE pair with two TALE fusions was more efficient than unmatched pairs with only one TALE fusion by merely 2.0-fold (60.7%/31%) or 7.5-fold (60.7%/8.1%). Apparently, the DddAtox N-terminal moiety fused to a TALE array bound to a half-site can recruit the DddAtox C-terminal moiety with no TALE array or vice versa to reconstitute a functional deaminase.
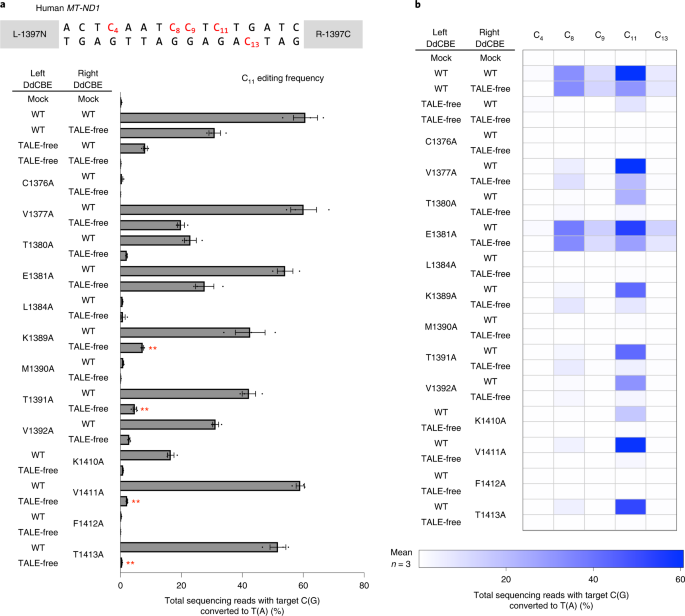
a, Base editing frequencies induced by G1397-split DddAtox interface mutants. The editing window and target cytosine bases are shown at the top. Plasmids encoding the mutant and wild-type (WT) or TALE-free DddAtox proteins were co-transfected as indicated in the left column. All of the TALE-fused and TALE-free constructs contain UGI. b, Heat map showing target C-to-T (G-to-A) editing efficiencies induced by DdCBE and the various mutants. Error bars are s.e.m. for n = 3 biologically independent samples. P = 0.0045, P = 0.0032, P = 0.0070 and P = 0.0066 for K1389A, T1391A, V1411A and T1413A, compared to wild-type and TALE-free pair, respectively. **P < 0.01, Student’s two-tailed t-test.
Source data
We also constructed the other DdCBE pair with DddAtox split at G1333 (L-1333N plus R-1333C), rather than G1397, specific to the MT-ND1 gene and tested whether L-1333N alone or R-1333C alone, each paired with the TALE-free DddAtox half split at G1333 (1333C or 1333N, respectively), could induce C-to-T edits. Again, each TALE fusion alone showed base editing at C8, with a frequency of 30.7% ± 1.7% (L-1333N) or 17.5% ± 0.8% (R-1333C), whereas the original DdCBE pair induced base edits at a frequency of 52.5% ± 3.2% (Fig. 3a). Thus, the original pair with two TALE fusions was more efficient than the unmatched pairs with one TALE fusion by merely 1.7-fold (52.5%/30.7%) or 3.0-fold (52.5%/17.5%). Taken together, these results suggest that DdCBEs can cause unwanted off-target mutations at sites where only one TALE array can bind. Because TALE proteins can bind to sites with a few mismatches18,19,20, DdCBE pairs probably induce many off-target mutations in the organelle or nuclear genome.

a, Base editing frequencies induced by G1333-split DddAtox interface mutants. The editing window and target cytosine bases are shown at the top. Plasmids encoding the mutant and wild-type (WT) TALE-free DddAtox proteins were co-transfected as indicated in the left column. The TALE-free DddAtox proteins used for the left and right DdCBEs were 1333N and 1333C, respectively. b, Heat map showing target C-to-T (G-to-A) editing efficiencies induced by DdCBE and the various mutants. Error bars are s.e.m. for n = 3 biologically independent samples. P = 0.0036, P = 0.0042 and P = 0.0034 for K1389A, T1391A and V1393A, compared to the wild-type and TALE-free pair, respectively. **P < 0.01, Student’s two-tailed t-test.
Source data
Split-dimer interface engineering of DddAtox
We sought to develop HiFi-DdCBEs that would not exhibit such off-target editing caused by spontaneous assembly of the split DddAtox halves. We reasoned that the split dimer interface could be engineered to inhibit or prevent self-assembly. To this end, we first identified amino acid residues in the interface of the two split DddAtox systems (split at G1333 and G1397). Because the crystal structure of DddAtox bound to DNA was not available, we had to rely on the three-dimensional (3D) structure of DddAtox complexed with DddI, the inhibitor protein (PDB ID: 6U08), in this analysis. We used a Python script in PyMOL (InterfaceResidues.py) to define the area of the surface of each split half and then to identify amino acid residues in each split half in close contact with those in the other split half. As a result, we chose nine amino acid residues in 1397N (the N-terminal DddAtox half split at G1397), four residues in 1397C (the C-terminal DddAtox half split at G1397), 14 residues in 1333Nand 15 residues in 1333C (Fig. 1b,c).
We then created a series of mutant DddAtox halves in which each of these amino acid residues was replaced with alanine (Fig. 1d,e), an amino acid residue with a chemically inert and non-bulky side chain. Then we measured the editing frequencies of these interface mutant DdCBEs in combination with a wild-type DdCBE partner or a TALE-free DddAtox half in HEK293T cells (Figs. 2 and 3). Many G1397-split DddAtox variants, containing interface mutations such as C1376A, M1390A and F1412A, failed to induce C-to-T conversions in the spacer region between the two TALE-binding sites, even when combined with the wild-type partner, suggesting that these mutants cannot interact with other wild-type DddAtox half nearby at the target site. Other DddAtox variants, such as those containing V1377A and E1381A, induced C-to-T edits at high frequencies in partnership with the TALE-free half, comparable to the wild-type DdCBE pair, showing that these mutations are neutral and do not prevent split dimer interactions.
Variants containing several mutations such as K1389A, T1391A, V1411A and T1413A were highly active when combined with the wild-type DdCBE partner but poorly active with the TALE-free partner. For example, the V1411A variant in combination with the wild-type partner showed an editing efficiency of 59.0% ± 1.1%, on par with the wild-type DdCBE pair (60.7% ± 3.2%), but showed an efficiency of 2.2% ± 0.1% with the TALE-free construct, demonstrating a 26.9-fold difference in discrimination (59%/2.2%) at the C11 position. As indicated above, the wild-type G1333-split DdCBE pair showed a 7.5-fold difference in discrimination (60.7%/8.1%). Furthermore, these variants were more selective than the wild-type DdCBE pair. Thus, these variants edited C11 preferentially over C8, C9and C13 in the editing window, whereas the wild-type DdCBE pair was much less discriminatory, editing all four cytosines with high frequencies of >6.6% (Fig. 2b).
We also obtained several desirable interface mutations in the DdCBE pair with DddAtox split at G1333 after screening 29 mutations (14 in 1333N and 15 in 1333C) (Fig. 3). Variants containing most of these mutations (such as I1299A, Y1316A, Y1317A and F1329A) were either poorly active even in combination with the wild-type partner or, in the case of other mutations (such as S1300A and T1314A), undesirably active in combination with the TALE-free partner. Notably, variants containing several mutations, including K1389A, T1391A and V1393A, were highly active when paired with the wild-type partner but inefficient when paired with the TALE-free partner. For example, the K1389A variant showed a 38.5-fold difference in discrimination (46.5% ± 3.6%/1.2% ± 0.02%), whereas the wild-type DdCBE pair showed a 3-fold difference in discrimination (52.5% ± 3.2%/17.5% ± 0.8%) at the C8 position. Furthermore, the pair containing the K1389A variant was more selective than the wild-type pair. Thus, this variant edited C8 preferentially over C9, C11 and C13, whereas the wild-type pair was promiscuous, editing all four cytosines with high frequencies of >19%. Note that C11 was preferentially edited by the pair containing V1411A in 1397C (Fig. 2b), whereas C8 was preferentially edited by the pair containing K1389A in 1333C (Fig. 3b). By contrast, both wild-type DdCBE pairs (DddAtox split at G1333 or G1397) exhibited poor discrimination. These results suggest that the interface mutants identified in this study can avoid unwanted bystander edits that are often observed with DdCBEs. To improve specificity further, we combined desired mutations to create double or triple interface mutants. Unfortunately, these mutants were either poorly efficient or inactive at the on-target site (Supplementary Fig. 1), suggesting that the resulting DddAtox halves cannot interact efficiently with the other halves even when brought together on target DNA by TALE proteins. We also found that our interface-engineered DdCBE pairs, without a nuclear export signal, could nevertheless reduce or avoid off-target mutations at highly homologous sites with a single nucleotide mismatch in the nuclear pseudogenes (Supplementary Fig. 2). We performed western blotting to compare the expression levels of the interface-engineered DdCBEs to that of the wild-type DdCBE in transfected cells and found that there was no significant difference (Supplementary Fig. 3), ruling out the possibility that the engineered variants were more specific because they were poorly expressed or unstable.
HiFi-DdCBEs avoid mitochondrial genome-wide off-target effects
Encouraged by these results, we conducted mtDNA-wide whole-genome sequencing to investigate whether the interface mutants can reduce off-target base editing in mitochondria. We chose several interface mutants, which were highly active when paired with the wild-type partner but inefficient when paired with the TALE-free partner (K1389A and T1391A in 1397N, V1411 and T1413A in 1397C, and K1389A, T1391A and V1393A in 1333C), transfected plasmids encoding each DdCBE pair into human cells, and then analyzed mtDNA isolated from the resulting cells. High-throughput sequencing of mtDNA isolated from untreated, control cells showed the presence of several naturally occurring single-nucleotide variants (SNVs) with a heteroplasmy fraction of ≥10%. After excluding these SNVs, we calculated average frequencies of mtDNA-wide off-target C-to-T editing in each sample with frequencies ≥1%. As shown in Fig. 4a, the wild-type DdCBE pair with DddAtox split at G1397 specific to the human MT-ND1 gene induced off-target mutations with an average C-to-T editing frequency of 0.0513% ± 0.0017%, which is 22.5-fold higher than the untreated control (0.0023% ± 0.0001). Strikingly, the TALE-free, G1397-split DddAtox pair also showed an average conversion frequency of 0.0485% ± 0.0031% in mtDNA, not much different from the wild-type DdCBE pair, indicating that an active deaminase can be formed by spontaneous assembly of the two inactive halves even in the absence of TALE–DNA interactions, which gives rise to collateral mutations. Furthermore, mismatched DdCBE pairs composed of a MT-ND1 TALE and a MT-ND4 TALE induced off-target editing with average mtDNA-wide conversion frequencies of 0.0198% ± 0.0111% (ND1-1397N + ND4-1397C) and 0.0083% ± 0.0067% (ND1-1397C + ND4-1397N), suggesting that they could form a functional DddA protein. Mismatched DdCBE pairs with a 1333-split also induced off-target editing with average conversion frequencies of 0.0513% ± 0.0199% (ND1-1333N + ND4-1333C) and 0.0261% ± 0.0161% (ND1-1333C + ND4-1333N). As expected, DdCBEs containing the catalytically deficient E1347A DddAtox mutant and a single DdCBE alone without its partner, used as negative controls, did not induce off-target editing in the mitochondrial genome (Supplementary Fig. 4). Notably, the DdCBE pair containing K1389A in 1397C demonstrated an average editing frequency of 0.0023% ± 0.0002%, essentially the same as the untreated control. Likewise, the DdCBE pairs containing T1391A in 1397C, V1411A in 1397N, or T1413A in 1397N showed 8.2- to 23.3-fold reduced off-target editing, compared to the wild-type DdCBE pair, with average off-target editing frequencies of 0.0022% ± 0.0001%, 0.0062% ± 0.0009%, and 0.0041% ± 0.0003%, respectively.
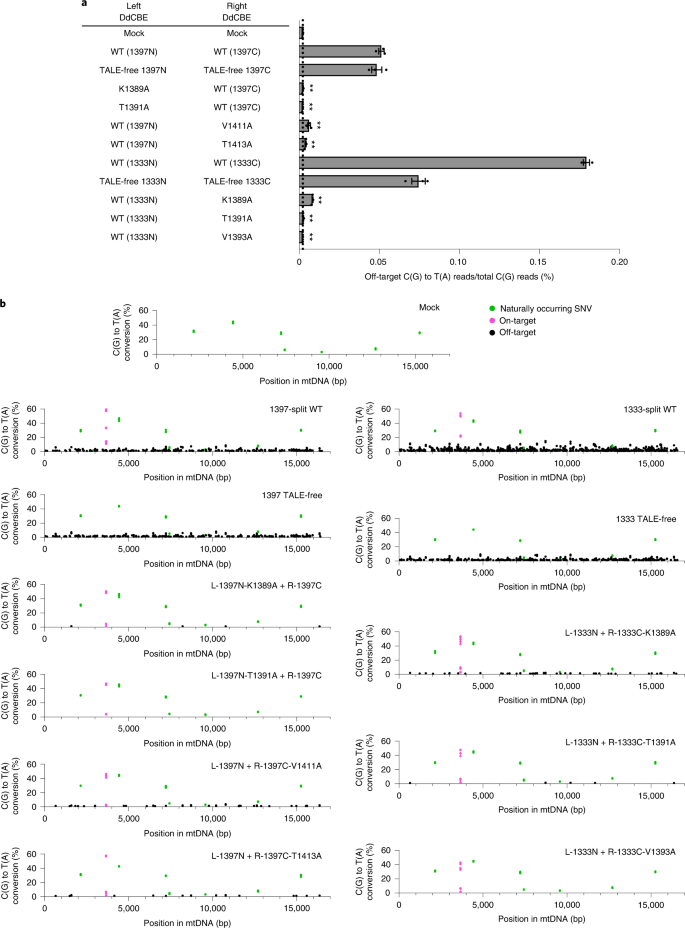
a, The average frequencies of mitochondrial genome-wide off-target editing induced by wild-type DdCBE, TALE-free constructs, and interface-engineered DddAtox pairs. Error bars are s.e.m. for n = 3 biologically independent samples. P = 0.0010, P = 0.0011, P = 0.0002, P = 0.0009, P = 0.0001, P = 0.0001 and P = 0.0001 for L-1397N-K1389A, L-1397N-T1391A, R-1397C-V1411A, R-1397C-T1413A, R-1333C-K1389A, R-1333C-T1391A and R-1333C-V1393A compared to the wild-type pairs, respectively. **P < 0.01, Student’s two-tailed t-test. b, Mitochondrial genome-wide plots for C-to-T point mutations with frequencies ≥1%. Naturally occurring SNVs, on-target edits (including bystander edits in the editing window) and off-target edits are shown in green, magenta and black, respectively. All data points from n = 3 biologically independent experiments are shown.
Source data
We next examined the number and position of off-target edits induced by various DdCBE constructs in the mitochondrial genome (Fig. 4b). The wild-type DdCBE pair and the TALE-free, G1397-split DddAtox pair caused off-target C-to-T edits at 238 and 224 sites, respectively, with conversion frequencies of ≥1.0%. Sequence logos obtained with DNA sequences around these off-target C-to-T edits showed a strong preference for the TC context (Supplementary Fig. 5), a signature of DddAtox substrate specificity1. The majority (>80%) of these off-target sites were co-edited by the DdCBE pair and the TALE-free split DddAtox pair (Supplementary Fig. 6a), indicating that DdCBE off-target editing is largely independent of TALE–DNA interactions. In sharp contrast, our interface variants induced off-target edits at merely 5 (K1389A), 0 (T1391A), 32 (V1411A) and 15 (T1413A) sites in the human mitochondrial genome. Note that the T1391A variant completely avoided off-target mutations.
We also compared the wild-type G1333-split DdCBE pair with variants containing alanine substitutions in the 1333C half. The wild-type DdCBE pair induced off-target C-to-T mutations with an average editing frequency of 0.1794% ± 0.0020%, significantly higher than that observed with the negative control. Notably, again, the TALE-free, G1333-split DddAtox pair also induced off-target mutations with an average frequency of 0.0745% ± 0.0042%. As expected, interface mutants reduced such off-target, collateral mutations in mtDNA. Thus, the DdCBE pair containing K1389A, T1391A or V1393A in 1333C showed 21- to 80-fold reduced off-target editing, compared to the wild-type DdCBE pair, with average off-target editing frequencies of 0.0084% ± 0.0003%, 0.0026% ± 0.0002% and 0.0022% ± 0.00003%, respectively. Mitochondrial genome-wide plots showed that the wild-type, G1333-split DdCBE pair and the TALE-free pair induced off-target edits at 640 and 328 sites, respectively (Fig. 4b and Supplementary Fig. 6b). The vast majority (97.6%) of the 328 off-target sites edited by the TALE-free pair were also edited by the G1333-split DdCBE pair, which induced off-target edits at a total of 320 additional sites. Presumably, these additional off-target edits were caused by nonspecific TALE–DNA interactions. DdCBE pairs containing our interface variants induced off-target edits at merely 51 (K1389A), 5 (T1391A) and 0 (V1393A) sites, largely or completely avoiding these collateral mutations.
Precision base editing in human mitochondrial genes with HiFi-DdCBEs
We next chose four different regions in the human mitochondrial genome (MT-ND4, MT-ND5, MT-ND6and MT-ATP8) to investigate whether our interface-engineered, HiFi-DdCBEs can achieve efficient C-to-T conversions at the four target sites without inducing collateral mutations (Fig. 5). As expected, the wild-type DdCBEs specific to these sites induced off-target mutations with average C-to-T editing frequencies that ranged from 0.0107% ± 0.0009% (MT-ND4) to 0.0180% ± 0.0005% (MT-ND5), which were 4.5- to 7.3-fold higher than than that for the untreated control (0.0024% ± 0.0001%). HiFi-DdCBEs containing interface-engineered DddAtox halves, however, largely avoided off-target base editing (Fig. 5 and Supplementary Figs. 7–10). For example, the ND4-specific DdCBE pair with the T1391A mutation in 1397N exhibited an average off-target editing efficiency of 0.0021% ± 0.0001%, a 5.0-fold (0.0107%/0.0021%) reduction, compared to the wild-type DdCBE. The pair with the K1389A mutation in 1333C showed a 6.0-fold (0.0150%/0.0025%) reduction, compared to the wild-type pair. For the other target sites (Fig. 5b–d), our HiFi-DdCBEs also demonstrated reduced off-target editing; for example, with average frequencies of 0.0110% (K1389A at MT-ND5) and 0.0024% (T1391A at MT-ND5) for the G1397-split system and 0.0041% (T1391A at MT-ND5) and 0.0026% (V1393A at MT-ND5) for the G1333-split system. In particular, HiFi-DdCBEs containing the T1391A variant specific to MT-ND4 or the T1413A variant specific to MT-ATP8 did not induce any off-target C-to-T edits in the human mitochondrial genome with frequencies of ≥1.0%, whereas wild-type DdCBEs induced a total of 63 and 86 off-target edits, respectively. The on-target editing efficiencies of these HiFi-DdCBEs were comparable with those of the wild-type DdCBEs (Supplementary Fig. 11).
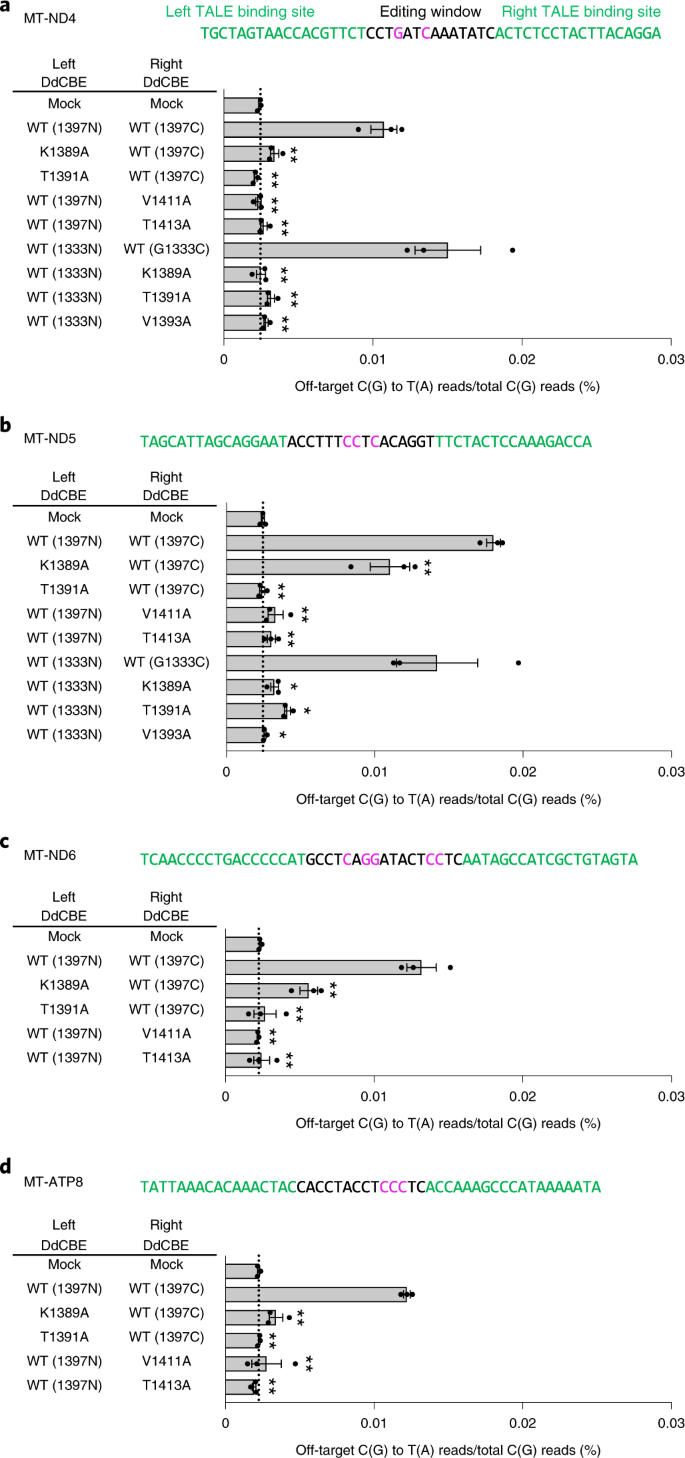
a–d,The MT-ND4 (a), MT-ND5 (b), MT-ND6 (c) and MT-ATP8 (d) sites targeted by DdCBE and interface-engineered variants. The bar graphs indicate the average frequencies of mitochondrial genome-wide off-target editing by wild-type and engineered DddAtox pairs. Error bars are s.e.m. for n = 3 biologically independent samples. *P < 0.05 and **P < 0.01, Student’s two-tailed t-test. Exact P values are provided in the source data.
Source data
Last, we introduced the interface mutations in DddA6 and DddA11, evolved DddA variants21, to create HiFi-DdCBEs with enhanced activity and broadened targeting scope, respectively. We found that DdCBEs containing DddA6 induced off-target mutations in the mitochondrial genome with average C-to-T editing frequencies that ranged from 0.0192% ± 0.0001% (MT-ND4) to 0.0459% ± 0.0041% (MT-ATP8), which were 8.1- to 20-fold higher than that for the untreated control (0.0024% ± 0.0001%). By contrast, HiFi-DdCBEs containing DddA6 largely avoided off-target base editing (Fig. 6 and Supplementary Figs. 12, 13). Thus, the ND4-specific DddA6-containing CBE pair with the K1389A mutation exhibited an average off-target editing efficiency of 0.0023% ± 0.0001%, an 8.5-fold (0.0192%/0.0023%) reduction, compared to the wild-type DddA6-containing pair. The DdCBE pair with the T1391A mutation in DddA6 also showed an 8.7-fold (0.0192%/0.0022%) reduction, compared to the DddA6-containing CBE pair. The ATP8-specific DddA6-containing CBE pair with the K1389A mutation exhibited an average off-target editing frequency of 0.0162% ± 0.0001%, a 2.8-fold (0.0459%/0.0162%) reduction compared to the wild-type DddA6-containing CBE pair. Likewise, the DdCBE pair with T1391A or V1411A in DddA6 demonstrated a 3.5-fold (0.0459%/0.0130%) or a 2.5-fold (0.0459%/0.0184%) reduction respectively, compared to the wild-type DddA6-containing CBE pair.
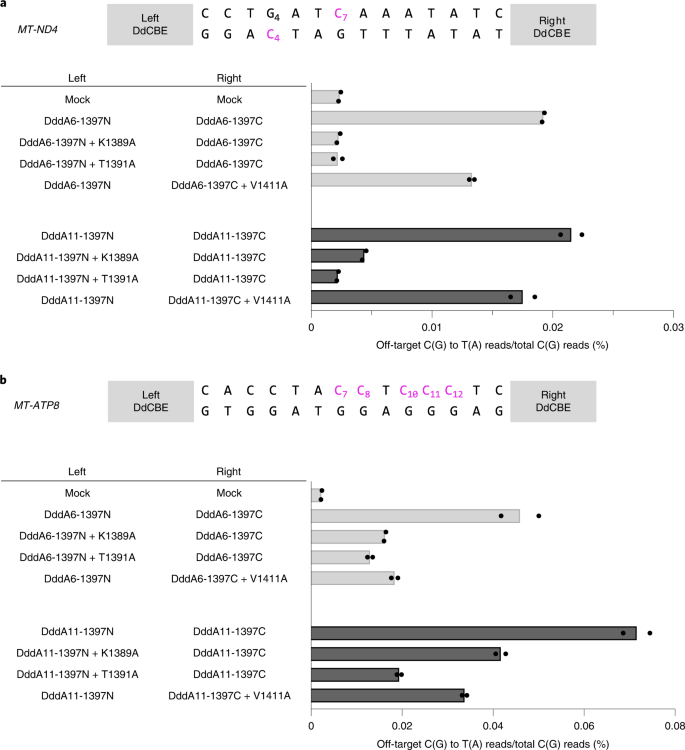
a,b, The average frequencies of mitochondrial genome-wide off-target editing induced by DddA6/DddA11 and interface-engineered variants targeting MT-ND4 (a) and MT-ATP8 (b) sites for n = 2 biologically independent samples. The editing windows are shown at the top and target cytosine bases are in magenta.
Source data
Furthermore, HiFi-DdCBEs containing DddA11 with interface mutations also largely avoided off-target base editing. DddA11-containing DdCBEs specific to two sites induced off-target mutations with average editing frequencies that ranged from 0.0215% ± 0.0009% (MT-ND4) to 0.0716% ± 0.0029% (MT-ATP8), which were 9.1- to 32-fold higher than that of the untreated control. By contrast, MT-ND4-specific HiFi-DdCBEs with interface mutations were highly specific, with average off-target editing frequencies of 0.0044% ± 0.0002% (K1389A), 0.0022% ± 0.0001% (T1391A) and 0.0175% ± 0.0010% (V1411A). Thus, these HiFi-DdCBEs showed a 4.9-fold (0.0215%/0.0044%), 9.8-fold (0.0215%/0.0022%) and 1.2-fold (0.0215%/0.0175%) reduction, respectively, compared to the DddA11-containing DdCBEs. Likewise, MT-ATP8-specific HiFi-DdCBEs with interface mutations demonstrated average off-target editing frequencies of 0.0417% ± 0.0011% (K1389A), 0.0194% ± 0.0005% (T1391A) and 0.0337% ± 0.0010% (V1411A). Thus, these HiFi-DdCBEs showed a 1.7-fold (0.0716%/0.0417%), 3.7-fold (0.0716%/0.0194%) and 2.1-fold (0.0716%/0.0337%) reduction, respectively, compared to the DddA11-containing DdCBEs. On-target editing efficiencies of these HiFi-DdCBEs were comparable with those of the wild-type DdCBE pairs containing DddA6 or DddA11 (Supplementary Fig. 14). Taken together, these results demonstrate that DdCBE off-target base editing in the mitochondrial genome can be largely avoided using our HiFi-DdCBEs with interface-engineered DddAtox split constructs.




